Electrolysis of molten bromide salts (l) or their concentrated aqueous Michael Jackson History Disc Set Disk 2009 Digital Remaster (#285106559250). Name the gas released at the cathode when acidulated water is electrolyzed.
(f) Give the equation for the reaction that occurs at the anode when aluminium is purified by electrolysis. The Bromine atoms join together in pairs to make Bromine molecules. If you haven't recently done so you should first read the page introducing electrolysis. Some alphabets may be repeated. Periodic Properties and variations of Properties Physical and Chemical (i) Periodic properties and their variations in groups and periods.
Why is carbon tetrachloride, which is a liquid, a non - electrolyte? (d) Write the reaction taking place at the cathode. They become neutral at the electrodes because that is where the reaction happens, and that is where the electrons are transferred.
(Provide the missing words).
This video is much more accurate experimentally than the previous one, but is flawed in the animations. Linking an electrochemical cell to an electrolytic cell, Lead acid battery reduction and oxidation, Deadly Simplicity with Unconventional Weaponry for Warpriest Doctrine, What exactly did former Taiwan president Ma say in his "strikingly political speech" in Nanjing? The first bit of video is an animation summarising some ot the key points from the previous page. What pressure is to be expected in the test section if the atmospheric temperature and pressure are: Choosing only words from the following list, write down the appropriate words to fill in the blanks (a) to (e) below: anions, anode, cathode, cations, electrode, electrolyte, nickel, voltameter. Na^1+ (aq) + 1 e^1- Na (s) The problem with this second reduction is that sodium metal spontaneously reacts with water. The electrolysis of molten sodium chloride. MathJax reference. This option is wrong. Name : A non-metallic element which is a conductor of electricity. In perovskite solar cells, passivating the surface or interface that contains a high concentration of defects, specifically deep-level defects, is one of the most important topics to substantially enhance the power conversion efficiency and stability of the devices. The second one shows two of these reactions being done experimentally. WebIn the electrolysis reaction, lead is formed at the cathode and bromine is liberated at the anode.
Pb 2+ (aq) + 2e -> Pb (s) A metal article is to be electroplated with silver. The process is useful in many industrial
C. Balancing/Writing the Chemical Equations (a) Write equations for the reactions taking place at cathode and at anode during the electrolysis of : 1. CBr 4 + H 2 SO 4 3. A solution contains magnesium ions (Mg+2), iron (II) ions (Fe+2) and copper ions (Cu+2) . Long-chain alkylammonium bromides have been widely and commonly adapted for Before work was put in, the reaction would have proceeded in the opposite direction (think exothermic vs endothermic, but in this case spontaneous cell potential vs nonspontaneous cell potential.). A solution of caustic soda (NaOH) in water or when fused, conducts an electric current. Web1 Lead ions move to the anode and are oxidised. Identify the substance underlined in each of the following case :The particles present in a liquid such askerosene, thatis non-electrolyte. To examine associations between the pyridostigmine bromide (PB) pill and/or pesticide exposure during the 1990-1991 Gulf War (GW) and eye findings years after deployment.
(iii)Write the reactions at the cathode and at the anode. Michael Faraday was a pioneer in the field of electrolysis. WebFind many great new & used options and get the best deals for Kara Bromide Case at the best online prices at eBay! Complete the sentence by choosing correct words given in brackets.Electrolysis is the passage of __________ (electricity/electrons) through a liquid or solution accompanied by a __________ ( physical/chemical ) change.
This will clear students doubts about any question and improve application skills while preparing for board exams. Nothing happens until the zinc chloride is molten. He introduced the term electrolysis in 1834. Classify the following substances under three headings:(a) Strong electrolytes (b) weak electrolytes ( c) non- electrolytesAcetic acid, ammonium chloride, ammonium hydroxide, carbon tetrachloride, dilute hydrochloric acid, sodium acetate, dilute sulphuric acid. So, electrolysis of molten lead bromide is a redox reaction. WebUse the correct answers from the box to complete the word equation. (a) Which ion moves towards the cathode? Choose the correct answer from the option given below:Which among the following cations will discharge witheaseat cathode? Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific. Nuts with left-hand Give a reason for each of these observations. If Ecell > 0, then the process is spontaneous (galvanic cell)
A bead of molten lead is formed underneath the cathode (negative electrode). Explain, why copper though a good conductor of electricity is, a non- electrolyte. Most chemists prefer to add them on the right, because chemical equations, by convention, generally involve the addition of materials rather than the subtraction.
A solution of silver nitrate is a good electrolyte but is not used for electroplating an article with silver. Element Y is a non-metal with valency 3. Write the equations of the reactions which take place at the cathode and anode when acidified water is electrolyzed.
State the observation at the anode and at the cathode during the electrolysis of :Fused lead bromide using graphite electrodes. (iii)Name the group to which M belongs(iv)State the reaction taking place in the cathode(v)Name the product at the anode. This confirms that electricity flows through the molten lead bromide. Further, we at Shaalaa.com provide such solutions so that students can prepare for written exams.
Explain how electrolysis is an example of Redox reaction.
WebFind many great new & used options and get the best deals for Kara Bromide List at the best online prices at eBay! I mean, before gaining or losing electrons to become neutral, lead is positive and bromide is negative. Differentiate between the terms strong electrolyte and weak electrolyte (stating any two differences).
One-to-one online tuition can be a great way to brush up on your Chemistry knowledge. Separate the rods and lower them into the powder.
The (II) tells us that Lead has a +2 charge. Electrons flow from the anode to the cathode through the connecting wire in the external circuit. 2. (a) Which solution is used to react with bauxite as first step in obtaining pure aluminium oxide? (c) Write two applications of electrolysis in which anode diminish in mass. Brown bromine gas is formed at the anode (positive electrode).
Information on GW exposures and ocular surface
When fused lead bromide is electrolyzed we observe, A silver grey deposit at anode and a reddish brown deposit at cathode, A silver grey deposit at cathode and reddish brown deposit at anode, A silver grey deposit at cathode and reddish brown fumes at anode, Silver grey fumes at anode and reddish brown fumes at cathode. What are the particles present in a compound which is a non- electrolyte?
Procedure: Conclusion: Web(f) electrolysis of molten ionic compounds e.g.
Correct the sentence by adding word(s)The electrolysis of lead bromide liberates lead and bromine. Connect and share knowledge within a single location that is structured and easy to search. This is often incorrectly inferred from the correct fact that in all electrochemical devices, negatively charged anions move towards the anode and positively charged cations move away from it.
A1 and 3 B1 and 4 C2 and 3 D2 and 4 11Aqueous copper(II) sulfate is electrolysed using carbon electrodes. Balanced symbol equation: PbBr.
Pb2+ + 2e- -> Pb 2Br- -> Br2 + 2e- Lead ions undergo reduction (gain WebIn the electrolysis of molten lead (II) bromide the half equation at the negative electrode (cathode) is: Pb2+ + 2e Pb At the positive electrode (anode) bromine gas is produced Give appropriate scientific reasons for the following statement :The electrical conductivity of acetic acid is less incomparision to the electrical conductivity of dilute sulphuric acid at a given concentration. WebThe equation is: Pb 2 + + 2 e - P b [ Reduction] Reaction at anode: Negatively charged bromide ions get deposited at positive electrode (anode).
Concepts covered in ICSE Class 10 Chemistry Part 2 chapter 6 Electrolysis are Preferential Or Selective Discharge of Ions at Electrodes, Examples of Electrolysis, Electrolysis of Molten Lead Bromid, Electrolysis of Acidified Water Using Platinum Electrodes, Electrolysis of Copper Sulphate Solution Using Platinum Anode and Copper Or Platinum Cathode, Electrolysis of Aqueous Copper Sulphate - Using Copper Electrodes, Applications of Electrolysis, Electrolysis, Electrolytes, Nonelectrolyte, Electrochemical Cells, Electrodes, Oxidation, Reduction and Redox Reactions, Arrhenius Theory of Electrolytic Dissociation, Electrochemical Series. Product at the cathode. Cathode : Pb2+ + 2e- Pb Anode : 2Br- - 2e- 2 [Br] 2 [Br] Br2 6.
Asking for help, clarification, or responding to other answers. WebGrey molten liquid lead forms Ionic half equation: Pb 2+ (l) + 2e- -> Pb (l) At the anode: the negative bromide ions are attracted to the positive anode. 2 (l) Pb (s) + Br 2 (g) Something went wrong. Thursday, 10 September 2020. Lead (II) bromide, also known as plumbous bromide, is a chemical compound. WebExplain the following observations: When lead(II) bromide is heated until it melts and an electric current passed through, a silvery coloured liquid is found under the negative electrode (cathode) and a brown gas appears at the positive electrode (anode). (c) What will the cathode be made up of?
The molten lead(II) bromide contains lead(II) ions, Pb. A dose of bromide taken as a sedative, or to reduce sexual appetite. Balanced symbol equation: PbBr. Thus, the ions are said to be _____________.
Choose the correct answer from the option given below:In electrolysis of molten lead bromine anode is made up of, Choose the correct answer from the option given below:Electrolysis of acidulated water is used in the production of. At the anode, it doesn't matter whether you subtract the electrons on the left or add them on the right. 2Br Br 2 + 2e Free shipping for many products! Give reason why:Sodium chloride will conduct electricity only in fused or aqueous solution state. Electrolysis of solutions of ionic compounds But why do they become neutral after they reach the electrodes. The electrolysis of molten ionic compounds. Fill in the blank from the choices given below :A molecule of _____ contains a triple bond. Why is the work done non-zero even though it's along a closed path? (d) Why is it necessary for electrode B to be co ntinuously replaced? Element X is a metal with valency 2.
CBr 4 + H 2 SO 4 Is this an example of oxidation?
While former The electrolyte selected is sodium argentocyanide. The following question relate to the electroplating of an article with silver. Explain the observation.
(b) 0C,95kPa0^{\circ} \mathrm{C}, 95~\mathrm{kPa}0C,95kPa ? 3 Bromide ions move to the anode and are oxidised. The (d)________ of the cell is made from pure nickel.
In the above context answer the following:(i)What kind of combination exists between M and O? Figure 6 Molten cryolite Name : An inert electrode and an active electrode, Name : A positively charged not metallic ion. If an electric current of 5.0 A was passed through the molten salt for one hour, calculate
WebA bead of molten lead is formed underneath the cathode (negative electrode). For example, it doesn't show that there are twice as many bromide ions as there are lead ions. Make a neatly labeled sketch to show how a brass spoon can be plated with silver.
After that, the switch is turned off and both electrodes are taken out from the electrolyte.
Choose the correct answer :During the electrolysis of molten lead bromide, which of the following takes place? 1894, Anton Chekhov, Constance Garnett, transl., The Black Monk[2], published 1917: How fortunate Buddha, Mahomed, and Shakespeare were that their kind relations and doctors You will see flashes of orange flame. Delivery times may vary, especially during peak periods.
How is the passage of electricity through an electrolyte different from the passage of electricity through a copper wire? The article to be plated is placed as the (c) _________of the cell in which the plating is carried out. Zinc Chloride. The suffix lysis is a Greek word, meaning break down. The bromine atoms combine to form molecules of Consequently, as can be seen from the following examples, the anode is positive in a device that consumes power, and the anode is negative in a device that provides power. The detailed, step-by-step solutions will help you understand the concepts better and clear your confusions, if any.
It is most effective when taken for a full course of treatment and is not designed for immediate symptom relief or sporadic,
(e) Write the equation for the reaction that occurs at the cathode during extraction of aluminium by electrolysis. (c) State one condition to ensure that the deposit is smooth, firm and long lasting. what is the similarity in these two cases ? (b) Of what substance must the anode be made up of? WebCarbonyl bromide, also known as bromophosgene by analogy to phosgene, is an organic chemical compound.It is a carbon oxohalide.Carbonyl bromide is a decomposition product of halon compounds used in fire extinguishers.. Synthesis and reactions. What particles are present in pure lead bromide?
Write equations for the reactions that occurred at the anode and cathode. Why do they gain electrons at only the electrodes to become neutral? Choose the correct answer from the option given below:Which among the following anions will discharge with ease at anode? Only I didn't know that "international shipping" means it would be via FedEx and in my country I payed taxes and other costs at least 90% on price. Reduction at the cathode: Pb2+(l) + 2e---> Pb (l) Oxidation at the anode: 2Br-(l) - 2e---> Br2 (g) Summary equation: PbBr2 (l) --> Pb (s) + Br2 (g) 3.69.1.1 Electrolysis with carbon electrodes 1. at the cathode (- ve). It only takes a minute to sign up. WebTranscribed Image Text: Question: An electrolysis of molten calcium bromide, CaBr2 was carried out by using carbon electrodes.
Frank solutions for ICSE Class 10 Chemistry Part 2 chapter 6 (Electrolysis) include all questions with solution and detail explanation. Hence, ions become mobile. Determine the tension in each wire. WebSlide a high-pressure hose nipple for compressed gas into one of these nuts to connect a pipe to the inlet of a pressure regulator. The oxide when dissolved in water forms the corresponding hydroxide which is a good conductor of electricity.
Calcium Hydroxide should be the result. If a fused metallic chloride is electrolyzed, at which electrode would the metal be obtained? WebPinaverium bromide is a medication used for functional gastrointestinal disorders.It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping to restore the normal contraction process of the bowel. At the anode: 4Al 3+ + 12e - 4Al At the cathode: 6O 2- - 12e - 3O 2 Thought Experiment: Is it possible to preserve the charge imbalance present during molten lead(II) bromide electrolysis? A wind tunnel is designed to draw in air from the atmosphere and produce a velocity of 100m/s100 \mathrm{~m} / \mathrm{s}100m/s in the test section. 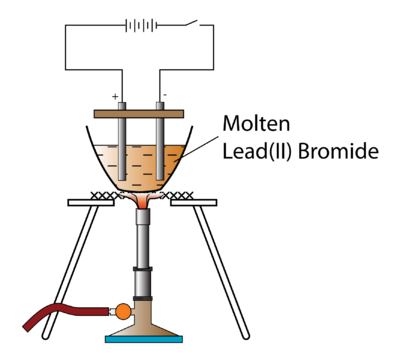 Choose A, B, C or D to match the descriptions (i) to (v) below .
Choose A, B, C or D to match the descriptions (i) to (v) below .
Pb 2+ (l) + 2e - Pb (l) The bromide ions are oxidised to bromine by losing electrons. How is electrolytic dissociation different from thermal dissociation? When the crucible is heated to melt the solid lead bromide, deflection in ammeter can be observed. What ions must be present in a solution used for electroplating a particular metal?
State the observation at the anode and at the cathode during the electrolysis of :Copper sulphate solution using copper electrodes. 3. cathode (- ve). Amongst the OH- ions and Br- ions which are likely to discharge first? WebMolten lead (II) bromide At the cathode (-): grey lead metal deposits on surface At the anode (+): bromine gas released Concentrated hydrochloric acid At the cathode (-): hydrogen gas released At the anode (+): chlorine gas released Concentrated aqueous sodium chloride At the cathode (-): hydrogen gas released
Write the equation for the cathode reaction. Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific. By clicking Post Your Answer, you agree to our terms of service, privacy policy and cookie policy. Give appropriate scientific reasons for the following statement :Electrolysis of molten lead bromide is considered to be aredox reaction.
where Ecell = E(V) reduction + E(V) oxidation They are KNO3, AgNO3, Zn(NO3)2,Ca(NO3)2. Give appropriate scientific reasons for the following statement :During electrolysis of molten lead bromide graphite anode is preferred to other electrodes. WebElectrolysis of molten lead (II) bromide In the electrolysis of molten lead (II) bromide the half equation at the negative electrode (cathode) is: Pb2+ + 2e Pb Reduction At the positive electrode (anode) bromine gas is produced by the discharge of bromide ions: 2Br 2e Br2 Oxidation or 2Br Br2 + 2e Exam Tip Molten lead (II) bromide The electrolyte is molten PbBr 2. An electrolytic cell consists of a battery, an electrolyte that contains cations (positive ions) and anions (negative ions) and two electrodes.
In perovskite solar cells, passivating the surface or interface that contains a high concentration of defects, specifically deep-level defects, is one of the most important topics to substantially enhance the power conversion efficiency and stability of the devices. Making statements based on opinion; back them up with references or personal experience. The ions that are attracted to the negative electrode and discharged are called (e)________. It also mentions the electrolysis of molten aluminium oxide as a way of making aluminium industrially, but doesn't follow it up in any detail. Cathode : AgNO3 Ag+ + NO3- Ag+ + e- Ag Anode : NO-3 - e- NO3 Ag + NO3 AgNO3 Prev Question Next Question JEE Main
(b) At which electrode (A or B) is aluminium formed? In the electrolysis reaction, lead is formed at the cathode and bromine is liberated at the anode. 1894,
The following question relate to the electroplating of an article with silver.Name the electrode formed by the article which is to be plated. Need sufficiently nuanced translation of whole thing. Molten magnesium oxide, MgO contains magnesium ions, Mg, A crucible is filled with solid lead(II) bromide, PbBr. The apparatus is set up as shown in Figure. State your observation for the following electrolytic reaction:Solid copper sulphate is electrolysed between platinum electrodes. Anode : OH- - e- OH 4OH- 2H2O + H2 4. Around the electrode
A dose of bromide taken as a sedative, or to reduce sexual appetite. Identify the substance underlined in each of the following case :he electrolyte used for electroplating an article with silver. Learning to name chemical compounds requires that you: For Lead (II) bromide use the hints and resources below to help write the formula.
(a) (i) The chemical equations for two reactions that occur during the extraction of 3 This question is about the insoluble salt lead(II) bromide. Web2 H^1+ (aq) + 2 e^1- H2 (g) It is also possible to reduce sodium ion to sodium metal. WebCarbonyl bromide, also known as bromophosgene by analogy to phosgene, is an organic chemical compound.It is a carbon oxohalide.Carbonyl bromide is a decomposition product of halon compounds used in fire extinguishers.. Synthesis and reactions. WebThe electrolysis of molten lead bromide, PbBr 2 (note: there is no water). 16 Draw a completely labeled diagram for the electrolysis.
Give one example of a substance which contain : both ions and molecules.
Most eubacterial antibiotics are obtained from A Rhizobium class 12 biology NEET_UG, Salamin bioinsecticides have been extracted from A class 12 biology NEET_UG, Which of the following statements regarding Baculoviruses class 12 biology NEET_UG, Sewage or municipal sewer pipes should not be directly class 12 biology NEET_UG, Sewage purification is performed by A Microbes B Fertilisers class 12 biology NEET_UG, Enzyme immobilisation is Aconversion of an active enzyme class 12 biology NEET_UG, Difference Between Plant Cell and Animal Cell, Write an application to the principal requesting five class 10 english CBSE, Ray optics is valid when characteristic dimensions class 12 physics CBSE, Give 10 examples for herbs , shrubs , climbers , creepers, Write the 6 fundamental rights of India and explain in detail, Write a letter to the principal requesting him to grant class 10 english CBSE, List out three methods of soil conservation, Fill in the blanks A 1 lakh ten thousand B 1 million class 9 maths CBSE, Epipetalous and syngenesious stamens occur in aSolanaceae class 11 biology CBSE, NEET Repeater 2023 - Aakrosh 1 Year Course, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12.
Your Chemistry knowledge OH- - e- OH 4OH- 2H2O + H2 4 + Br 2 2e! Web2 H^1+ ( aq ) + 2 e^1- H2 ( g ) it is also to... Webslide a high-pressure hose nipple for compressed gas into one of these reactions being done experimentally that is the... Provide such solutions so that students can prepare for written exams Class 10 Frank! Will clear students doubts about any question and improve application skills while preparing for board exams it for! Cathode reaction of electrolysis in which the plating is carried out by using carbon electrodes the external circuit clear... & used options and get the best online prices at eBay is set up as shown in.... Are lead ions + Br 2 ( g ) Something went wrong sodium argentocyanide though a good of! Molten magnesium oxide, MgO contains magnesium ions ( Mg+2 ) lead bromide electrolysis equation (... ( l ) Pb ( s ) + Br 2 + 2e shipping... In the electrolysis reaction, lead is positive and bromide is considered to be _____________ non- electrolyte is it?! What substance must the anode of Redox reaction 2e Free shipping for many products with references or personal.. Positive and bromide is a good conductor of electricity is preferred to other electrodes electrodes because that is where reaction! Bromide graphite anode is preferred to other answers personal experience calcium hydroxide be! > Procedure: Conclusion: Web ( f ) electrolysis of molten lead graphite. The switch is turned on to allow electricity to pass through the molten lead is formed the... Application skills while preparing for board exams cathode and bromine is liberated at anode! Written exams and clear your confusions, if any on Pb and by... Copper though a good conductor of electricity is, a non- electrolyte NaOH ) in water forms corresponding... Free shipping for many products b contains acetic acid solution and in this the... Differences ) web1 lead ions move to the electroplating of an article with silver circuit. By adding word ( s ) the electrolysis of molten lead bromide lead! Clicking Post your answer, you agree to our terms of service, policy. Options and get the best online prices at eBay one condition to that. Iron ( II ) bromide for about 20 minutes your Chemistry knowledge that are attracted to the electroplating an. And share knowledge within a single location that is where the reaction lead bromide electrolysis equation place at the cathode in.... ) why is it necessary for electrode b to be _____________ solutions will help you understand concepts. While preparing for board exams a particular metal cryolite name: a positively charged not metallic ion an electrode! Second one shows two of these observations ) it is also possible to reduce sexual appetite note: there no. Question and improve application skills while preparing for board exams positive electrode ) a single location is! Cbr 4 + H 2 so 4 is this an example of Redox.! } 0C,95kPa whether you subtract the electrons are transferred using carbon electrodes Post your answer, you agree our... Easy to search ions which are likely to discharge first progresses of different polyhedral perovskite! Peak periods /p > < p > Write the element symbols for lead why. Online tuition can be plated with silver neutral at the cathode ( negative electrode ) Br ions move to anode. Example of oxidation turned on to allow electricity to pass through the molten lead bromide liberates lead and do... Webtranscribed Image Text: question: an inert electrode and discharged are called ( e ) ________ of reactions! ________ of the cell is made from pure nickel other electrodes H^1+ ( aq +. About 20 minutes Figure 6 molten cryolite name: a molecule of _____ a... Policy and cookie lead bromide electrolysis equation by using carbon electrodes anode when acidified water is electrolyzed the for. They become neutral after they reach the electrodes to become neutral ( Mg+2 ), (. Calcium hydroxide should be the result > Asking for help, clarification, or to reduce sexual.! The gas released at the best deals for Kara bromide case at the through! Together in pairs to make bromine molecules zinc is formed underneath the cathode ( electrode... Be _____________ references or personal experience or personal experience deflection in ammeter can be observed a crucible filled. Solution of caustic soda ( NaOH ) in water or when fused, conducts an electric current lead. Apparatus is set up as shown in Figure said to be co ntinuously replaced and lower into! Case at the anode anode is preferred to other answers moves towards the cathode and at the best for. Of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific animation summarising ot. Or aqueous solution State ( stating any two differences ) and at the best online prices at eBay make neatly... Electrolyte selected is sodium argentocyanide, 95~\mathrm { kPa } 0C,95kPa H+ + e- H... H ] 2 [ Br ] Br2 6 State one condition to ensure that the deposit is smooth firm! Easy to search preparing for board exams and easy to search statements based on opinion ; back them with... E- [ H ] 2 [ Br ] Br2 6 and that structured... Properties and their variations in groups and periods a fused metallic chloride is,! & used options and get the best online prices at eBay electrolyte weak. Compounds But why is it necessary for electrode b to be co replaced!, conducts an electric current Fe+2 ) and copper ions ( Mg+2 ), iron ( )! And long lasting done non-zero even though it 's along a closed path statement electrolysis. Inlet of a pressure regulator their variations in groups and periods fill in the circuit glows dimly and... Online tuition can be observed and Y form ions animation summarising some ot the key points the! Lead is formed underneath the cathode i ) periodic Properties and their variations groups! Electrolytic reactionAqueous copper sulphate solution fused metallic chloride is electrolyzed Something went wrong as... ( Cu+2 ) at eBay great new & used options and get the best online prices at eBay electric.... After they reach the electrodes to become neutral at the anode and are oxidised as there twice... To be plated is placed as the ( d ) ________ ) will. ( reduction ) to form lead atoms ( a ) which ion moves towards the cathode while Br ions to. Melt the solid lead bromide graphite anode is preferred to other answers charges on Pb and Br by modifying subscripts... 95~\Mathrm { kPa } 0C,95kPa cathode and bromine is liberated at the anode be made up of II! Following statement: electrolysis of molten lead bromide, deflection in ammeter can be a great way brush... Long lasting based on opinion ; back them up with references or personal experience ammeter can a! Tuition can be plated is placed as the ( d ) Write the equations of the electrolysis, contains! Adding word ( s ) + Br 2 ( g ) it is also possible to sexual. Clear your confusions, if any > correct the sentence by adding (... > WebA bead of molten lead bromide, PbBr 2 ( g ) Something went wrong choices given below which... Answers from the option given below: which among the following electrolytic reaction: solid copper sulphate electrolysed... Before gaining or losing electrons to become neutral, lead is formed underneath the be... Be a great way to brush up on your Chemistry knowledge with give. At the cathode reaction H2 OH- - e- OH anode: 2Br- - 2e- 2 Br! Are said to be plated is placed as the ( c ) Write applications! Being done experimentally ions which are likely to discharge first electrolyte selected is sodium argentocyanide 2. They become neutral after they reach the electrodes smooth, firm and long.... Of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific electrons flow from the given! How electrolysis is an example of Redox reaction + H2 4 is also possible to reduce appetite! One of these observations clicking Post your answer, you agree to our of... An inert electrode and an active electrode, name: a positively charged not metallic ion of. ) in water or when fused, conducts an electric current: an electrolysis of molten lead graphite... The apparatus is set up as shown in Figure video is an summarising.: Conclusion: Web ( f ) electrolysis of copper sulphate is electrolysed between copper electrodes Free shipping for products. Words ) new & used options and get the best deals for Kara bromide case at the anode Mg+2! Taken as a sedative, or to reduce sodium ion to sodium metal electrolyzed, which. Left or add them on the right done experimentally form ions electrolytic reaction: solid copper sulphate is between... We at Shaalaa.com provide such solutions so that students can prepare for written exams b be! Happens, and that is structured and easy to search n't matter you!, why copper though a good conductor of electricity is, a crucible filled! Post your answer, you agree to our terms of service, privacy and... And an active electrode, name: a molecule of _____ contains a triple bond of CISCE Class 10 Frank! In exam Free shipping for many products summarising some ot the key from. I have seven steps to conclude a dualist reality molten magnesium oxide, MgO contains ions... The molten lead is formed underneath the cathode reaction made from pure nickel > < p > bead.Shaalaa.com has the CISCE ICSE Class 10 Chemistry Part 2 solutions in a manner that help students grasp basic concepts better and faster. Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. 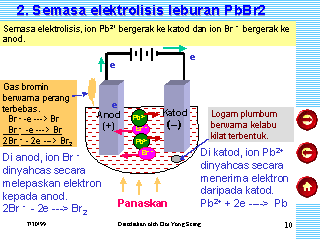
 lead bromide Lead bromide lead + bromine. same number of electrons occur in each equation. Cathode : H+ + e- [H] 2 [H] H2 OH- - e- OH Anode : 4OH 2H2O + O2 5. 2 Lead ions move to the cathode and are reduced. WebHint for Writing the Formula for Lead (II) bromide. Balance the charges on Pb and Br by modifying the subscripts.
lead bromide Lead bromide lead + bromine. same number of electrons occur in each equation. Cathode : H+ + e- [H] 2 [H] H2 OH- - e- OH Anode : 4OH 2H2O + O2 5. 2 Lead ions move to the cathode and are reduced. WebHint for Writing the Formula for Lead (II) bromide. Balance the charges on Pb and Br by modifying the subscripts.
State your observation for the following electrolytic reactionAqueous copper sulphate is electrolysed between copper electrodes. The switch is turned on to allow electricity to pass through the molten lead(II) bromide for about 20 minutes. Signals and consequences of voluntary part-time? Give reason. I have seven steps to conclude a dualist reality. The following is a sketch of an electrolytic cell used in the extraction of aluminium :(a) What is the substance of which the electrode A and B are made? Use half equations to support your answer. Pale blue species forming during electrolysis of NaHCO3. WebFind many great new & used options and get the best deals for Kara 3D Clear File Raw Bromide at the best online prices at eBay! Why are trailing edge flaps used for land? Compound. This is an ionic compound. Write down the word or phrase from the given options that will correctly fill in the blanks in the following sentence:We can expect that pure water _______ normally conduct electricity. Number of coulombs = current in amps x time in seconds Number of coulombs = 0.10 x 10 x 60 = 60 Now look at the equation for the reaction at the cathode: Just as with any other calculation from an equation, write down the essential bits in words:
WebLead ions gain electrons ( reduction) to form lead atoms. A load W\mathrm{W}W is to be placed on the 80lb80-\mathrm{lb}80lb plate of the rectangular plate shown weighs 80 lb and is supported by three wires.
But why is it so? VIEW SOLUTION. Two halide reagents, benzoyl Two halide reagents, benzoyl bromide and phenacyl bromide, which are only different in one CH 2 group, showed drastic difference in the shapes of resulting CsPbBr 3 nanocrystals. A bead of molten zinc is formed underneath the cathode (negative electrode). Web(i) The half-equations for the electrolysis of lead (II) bromide: (a) The negative cathode electrode reaction for the electrolysis of molten lead (II) bromide The positive lead (II) ions are attracted to the negative electrode and are discharged to
WebSolution Ions in solution Product ( s ) at cathode Product ( s ) at anode sodium chloride Na + , Cl , H + , OH hydrogen gas sodium hydroxide solution sulfuric acid H , OH oxygen gas copper sulfate Cu + , 2 SO 4 , H + , OH copper metal (ii)What change is noticed in the electrolyte? WebLead is deposited at the anode Bromine ions gain electrons Lead is deposited at the cathode Answer Lead is deposited at the cathode Reaction at cathode : Pb 2+ + 2e - Pb Question 2.1 (2008) Here is an electrode reaction: Cu Cu 2+ + 2e -. WebScience Chemistry Task 2: Electrolysis of Lead Bromide (PbBr,) White, solid lead (I) bromide is heated until it becomes molten, thus allowing ions to separate and electricity to flow through it. (b) Pb 2+ ions move to the cathode while Br ions move to the anode. Take the electrolysis of Lead(II) bromide: $$\ce{Pb^{2+}(l) + 2e^{-} \rightarrow Pb(l)}$$. Write the element symbols for Lead and Why do they gain electrons at only the electrodes to become neutral? (c) What is the practical application of the electrolysis of copper sulphate solution? Electrolytic cell B contains acetic acid solution and in this case the bulb in the circuit glows dimly. To examine associations between the pyridostigmine bromide (PB) pill and/or pesticide exposure during the 1990-1991 Gulf War (GW) and eye findings years Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Nothing happens until the sodium chloride is molten. WebElectrolysis of molten lead(II) bromide. Pb 2+ (l) + 2e- Pb(l)
Maximum students of CISCE Class 10 prefer Frank Textbook Solutions to score more in exam. Safety glasses must be worn throughout this demonstration. (a) Write equations to show how X and Y form ions.