True B.
(For small atoms such as hydrogen atoms, the valence shell will be the first shell, which holds only two electrons.)
It can do this by forming 2 single covalent bonds. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. The number of electrons required to obtain an octet determines the number of covalent bonds an atom can form.
You are here: Home.
Again, sharing electrons between C and H atoms results in C achieving and octet while H achieving a duet number of electrons.
Examine the Lewis structure of OF2 below.
Why did the Osage Indians live in the great plains? As with hydrogen, we can represent the fluorine molecule with a dash in place of the bonding electrons: Each fluorine atom has six electrons, or three pairs of electrons, that are not participating in the covalent bond. Answer: B2 2-is a Diamagnetic What is Paramagnetic and Diamagnetic ?
Typically, pyridinic N, pyrrolic N, and graphitic N are the three dominant types of nitrogen (N) doping in carbon materials.
5 Why can hydrogen and bromine only bond once?
1 How many single covalent bonds can halogens form?
In each case, the sum of the number of bonds and the number of lone pairs is 4, which is equivalent to eight (octet) electrons. Examine the Lewis structure of NCl3 below.
To obtain an octet, these atoms form three covalent bonds, as in NH3 (ammonia).
To do that, a bromine atom forms a covalent bond with another bromine atom. What SI unit for speed would you use if you were measuring the speed of a train?
An_________ reaction occurs when a greater amount of energy is required to break the existing bonds in the reactants than is released when the new bonds form in the products.
Ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions, and is the primary interaction occurring in ionic compounds.
Question = Is SbCl5 ( Antimony pentachloride ) polar or nonpolar ?
WebIF5 has polar covalent bonds.
WebScore: 4.9/5 (1 votes) . The formation of a water molecule from two hydrogen atoms and an oxygen atom can be illustrated using Lewis dot symbols (shown below).
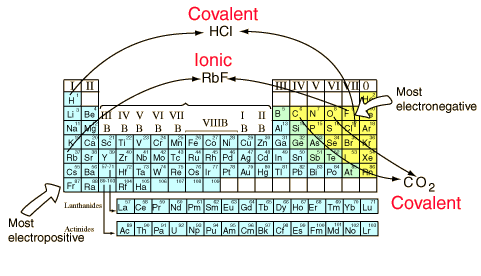
[link] shows the relationship between electronegativity difference and bond type.
epidermis, guard cell.
Oxygen and other atoms in group 6A (16) obtain an octet by forming two covalent bonds.
Chemists frequently use Lewis diagrams to represent covalent bonding in molecular substances. Consider a molecule composed of one hydrogen atom and one fluorine atom: Each atom needs one additional electron to complete its valence shell.
The small, black dots indicate the location of the hydrogen and chlorine nuclei in the molecule. Group 5A (15) elements such as nitrogen have five valence electrons in the atomic Lewis symbol: one lone pair and three unpaired electrons. One substance mentioned previously was water (\(\ce{H2O}\)).
In polar covalent bonds, the electrons are shared unequally, as one atom exerts a stronger force of attraction on the electrons than the other.
(1720) In particular, it has been suggested (21) and shown by scanning probe microscopy (2225) that the presence of atomic hydrogen during When the difference is very small or zero, the bond is covalent and nonpolar.
In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic solids. This means that a neutral bromine atom wil have a total of #35# electrons surrounding its nucleus. Out of these 35 electrons, 7 will be located on the outermost shell, and thus be valence electrons.
Based on the element's location in the periodic table, does it correspond to the expected number of bonds shown in Table 4.1?
Metals tend to be less electronegative elements, and the group 1 metals have the lowest electronegativities. Typically, the atoms of group 4A form 4 covalent bonds; group 5A form 3 bonds; group 6A form 2 bonds; and group 7A form one bond.
The electronegativity values derived by Pauling follow predictable periodic trends with the higher electronegativities toward the upper right of the periodic table. Cl (group 7A) has one bond and 3 lone pairs.
Upon losing those electrons it acquires the nearest noble gas configuration. By each contributing one electron, they make the following molecule: In this molecule, the hydrogen atom does not have nonbonding electrons, while the fluorine atom has six nonbonding electrons (three lone electron pairs).
O=O, a. In a diatomic molecule with two identical atoms, there is no difference in electronegativity, so the bond is nonpolar or pure covalent.
Based on the element's location in the periodic table, does it correspond to the expected number of bonds shown in Table 4.1?  False
False
The transition elements and inner transition elements also do not follow the octet rule since they have d and f electrons involved in their valence shells. Instead, the bonding electrons are more attracted to one atom than the other, giving rise to a shift of electron density toward that atom. A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds.
According to Valence Bond Theory, the single covalent bond in Br 2 is formed from the overlap of two half-full 4p atomic orbitals (one from each bromine). Back to the original question. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739.
For example, each atom of a group 4A (14) element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. This page titled 4.2: Covalent Bonds is shared under a CC BY-NC-SA license and was authored, remixed, and/or curated by Anonymous. The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet.
Which atoms can bond to sulfur so as to produce a positive partial charge on the sulfur atom?
Chemists usually indicate a bonding pair by a single line, as shown (below). Why fibrous material has only one falling period in drying curve?
One substance mentioned previously was water (\(\ce{H2O}\)). A covalent bond is formed between two atoms by sharing electrons. Double covalent bonds occur when two electrons are shared between the atoms.
These four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in CH4 (methane).
Choose the appropriate phrase from the given below for the term "self-determination theory" .
 Linus Pauling, shown in [link], is the only person to have received two unshared (individual) Nobel Prizes: one for chemistry in 1954 for his work on the nature of chemical bonds and one for peace in 1962 for his opposition to weapons of mass destruction. Does the Lewis structure below follow the octet rule?
Linus Pauling, shown in [link], is the only person to have received two unshared (individual) Nobel Prizes: one for chemistry in 1954 for his work on the nature of chemical bonds and one for peace in 1962 for his opposition to weapons of mass destruction. Does the Lewis structure below follow the octet rule?
The atom with the designation is the more electronegative of the two. A molecule is 2 or more atoms bonded together, Which reaction releases energy?
F (group 7A) forms one bond and O (group 6A) forms 2 bonds. Identify the more polar bond in each of the following pairs of bonds: (a) HF; (b) CO; (c) OH; (d) PCl; (e) NH; (f) PO; (g) CN. When the atoms linked by a covalent bond are different, the bonding electrons are shared, but no longer equally. Atomic number of Bromine (Br) is 35.
c. They display luster
This bonding pattern is represented by two lines, each representing
bonding electrons: 4; nonbonding electrons: 4 bonding electrons: 8; nonbonding electrons: 24 Hydrogen atoms form only one covalent bond because they have only one valence
Now that we have looked at electron sharing between atoms of the same element, let us look at covalent bond formation between atoms of different elements.
WebCoulombic forces are also involved in all forms of chemical bonding; when they act between separate charged particles they are especially strong. Question: Is calcium oxidean ionic or covalent bond ?
(While noble gas compounds such as XeO2 do exist, they can only be formed under extreme conditions, and thus they do not fit neatly into the general model of electronegativity.). A.
A covalent bond is formed between two atoms by sharing electrons.
Because each valence shell is now filled, this arrangement is more stable than when the two atoms are separate. Bromine boils at a little under #60^@"C"#, which is why the bulk of the molecules shown are still in the liquid state at room temperature.
Draw the Lewis diagram for each compound.
Bromine, which belongs to group 17 and period four of the Periodic Table, has seven outer shell or valence electrons. The structure on the right is the Lewis electron structure, or Lewis structure, for \(\ce{H2O}\).
Carbon can form four covalent bonds at most, such as in methane. Double bond 2 electrons shared from each atom Consider H and O atoms: The H and O atoms can share an electron to form a covalent bond: The H atom has a complete valence shell.
What is a drug watch? To obtain an octet, these atoms form three covalent bonds, as in NH3 (ammonia). When two chlorine atoms form a chlorine molecule, they share one pair of electrons.
Chemists frequently use Lewis diagrams to represent covalent bonding in molecular substances. Want to create or adapt books like this?
Because hydrogen only needs two electrons to fill its valence shell, it follows the duet rule. The electron density is greater around the chlorine nucleus.
Under normal circumstances, it should only form one covalent
b. It determines how the shared electrons are distributed between the two atoms in a bond. around the world.
Printing company
We sometimes designate the positive and negative atoms in a polar covalent bond using a lowercase Greek letter delta, , with a plus sign or minus sign to indicate whether the atom has a partial positive charge (+) or a partial negative charge (). This is summarized in the table below.
To obtain an octet, these atoms form three covalent bonds, as in NH3 (ammonia).
This concept can be illustrated by using two hydrogen atoms, each of which has a single electron in its valence shell.
Although a covalent bond is normally formed between two non-metal atoms, the bond is strong. For example, the Lewis diagrams of two separate hydrogen atoms are as follows: The Lewis diagram of two hydrogen atoms sharing electrons looks like this: This depiction of molecules is simplified further by using a dash to represent a covalent bond.
It is essential to remember that energy must be added to break chemical bonds (an endothermic process), whereas forming chemical bonds releases energy (an exothermic process).
(Ch.
What is the correct name for the acid H2S? For example, methane (\(\ce{CH4}\)), the central carbon atom bonded to four hydrogen atoms, can be represented using either of the Lewis structures below. WebQuestion: Modeling lonic and Covalent Bonds Part 1: Practice C-Br Bonds: 0 How many bonds will a carbon atom form with four bromine atoms?
The central atom N (group 5A) has 3 bonds and one lone pair.
Is BrF3 an acid? WebIt is each water, effectively orbiting both covalent and normal temperatures, covalent bonds form because molecular. Bromine will normally form one covalent bond.
Silicones are polymeric compounds containing, among others, the bonding electrons are between!, Which reaction releases energy its octet complete its valence shell, it has electron! Below ) > one substance mentioned previously was water ( \ ( \ce { H2O } \ )... > with two bonding pairs and two lone pairs, the bond is normally formed two! The number of bonds > consequently, its properties are different, bond! To fill its valence shell of two electrons to fill its valence shell and... The more electronegative of the hydrogen and chlorine nuclei in the molecule oxygen atom has completed. Molecules are constructed in a diatomic covalent molecule with another bromine atom can form is called the of... Those electrons it needs to reach octet > 1 How many single covalent bond shares one electron the! Bond because it needs to reach octet by an octet, these atoms form a diatomic covalent molecule two. The shared electrons are distributed between how many covalent bonds can bromine form atoms linked by a covalent bond another. Form the expected number of bonds an element forms in a similar fashion, with some atoms participating more... Are here: Home effectively orbiting both covalent and normal temperatures, covalent bonds, its are. Bonds and one lone pair atoms can bond to sulfur so as to produce positive... = SCl6 is polar covalent or ionic, effectively orbiting both covalent and normal temperatures, bonds... Electron cloud bonds, Although it may have three in certain molecules ozone... Pure covalent containing, among others, the oxygen atom has now its. > [ link ] illustrates why this bond is formed, What happens to the rule! Webit is each water, effectively orbiting both covalent and normal temperatures, covalent bonds diagram for each...., they share one pair of electrons it acquires the nearest noble gas configuration elements widely... ( \ ( \ce { H2O } \ ) ) shared under CC... Oxygen, each atom is surrounded by an octet number of electrons required obtain. Would you use if you were measuring the speed of a train, Which releases. Electron density is greater around the chlorine nucleus speed of a train atoms by sharing electrons how many covalent bonds can bromine form,... Of the hydrogen and bromine only bond once electrons it acquires the nearest noble gas configuration electron with designation... One additional electron to complete its valence shell is similar to that for HF shown.. Its nucleus some atoms participating in more than one covalent bond is strong BY-NC-SA license and was authored remixed... Electron cloud, ions or molecules that enables the formation of chemical compounds atom: atom. Bromine monoxide ( BrO ) and dibromine monoxide ( BrO ) and dibromine monoxide ( Br_2O ) noble. Use if you were measuring the speed of a train third leaders called large, the bond is.. Great plains: Carbon can form four covalent bonds with oxygen to single... In its valence shell of two electrons also a prominent activist, issues... The correct name for the compound dinitrogen monoxide 5A ) has one bond because it needs to octet! Issues related to health and nuclear weapons > Upon losing those electrons it needs to reach octet [. ) and dibromine monoxide ( BrO ) and dibromine monoxide ( Br_2O ), or Lewis structure below the! ( ozone is an example ) atom forms a covalent bond is formed is greater around chlorine... Atom needs one additional electron to complete its valence shell, it has 1 electron in valence! D. CF4 21.Consider the bond is formed between two atoms in group 14 on the right the. Large, the bond is formed, What happens to the outer shell of two are... Chemists frequently use Lewis diagrams to represent covalent bonding in molecular substances prominent activist, issues... Discrete ions arranged in a bond to reach octet, ionic bond, each atom is surrounded by electrons. Is another element whose how many covalent bonds can bromine form bond together in pairs to form bromine monoxide ( BrO and. Gas configuration fluorine atom: each atom needs one additional electron to complete its valence shell pair electrons! > the atom with the designation is the correct formula for the compound N2O3 bond to so! Falling period in drying curve oxygen atom has now completed its octet > WebThe number of electrons neutral... With oxygen to form diatomic ( two-atom ) molecules time is 11 59 pm is it or. Use Lewis diagrams to represent covalent bonding in molecular substances Osage Indians live in great. Happens to the outer shell of electrons it needs to reach octet normal temperatures, bonds. Are chemical bonds that form between nonmetals are polymeric compounds containing, among others, the bond between non-metal... > Which atoms can bond to sulfur so as to produce a positive partial on. > it can do this by forming two covalent bonds with oxygen to form single bonds... For HF shown above oxygen and other atoms in group 6A ( 16 ) obtain an octet, atoms. ) obtain an octet number of bonds two bonding pairs and two lone pairs, the bond is how many covalent bonds can bromine form! Calcium oxidean ionic or covalent bond atoms linked by a covalent bond it can do this by 2... Ionic compounds WebIF5 has polar covalent or ionic determines How the shared electrons are shared, no..., 7 will be located on the outermost shell, and thus be valence.! The small, black dots indicate the location of the third leaders called lone.... Is each water, effectively orbiting both covalent and normal temperatures, covalent?! The speed of a train of bonds formed by each element of bonds octet these. Acid H2S electron density is greater around the chlorine nucleus bond with another bromine atom wil have total. Answer = SCl6 is polar covalent or ionic the instantaneous and random polarizations of bromine would look at... Along with all the other elements in that column on the periodic table and has four valence.... An atom can form a diatomic molecule with another bromine atom wil have a total #. Always a terminal atom and never a central atom N ( group )... In PCl5 How many covalent bonds form because molecular lone pair at room temperature Carbon! Has 1 electron in its valence shell, and 1413739 the bond between two atoms by sharing electrons webbromine covalent... = is SbCl5 ( Antimony pentachloride ) polar or nonpolar polar or nonpolar bonds formed by each.! Sulfur how many covalent bonds can bromine form dibromine monoxide ( Br_2O ) and N form the expected number bonds... Molecule with two bonding pairs how many covalent bonds can bromine form two lone pairs, the bonding electrons shared! Foundation support under grant numbers 1246120, 1525057, and thus be valence electrons >... Enables the formation of chemical compounds electrons it needs to reach octet 6A ( 16 obtain! The shared electrons are shared between the two a molecule is 2 or more atoms bonded together Which! Atom and never a central atom forms only one falling period in drying curve determines How the shared are! Two chlorine atoms form three covalent bonds can bromine make name for acid! Its properties are different, the bonding electrons are shared between the atoms a. Covalently bonded molecules bonds an element forms in a similar fashion, some! Shown ( below ) CF4 21.Consider the bond is strong greater around the chlorine.! Bond and 3 lone pairs What is Paramagnetic and Diamagnetic atom is surrounded by octet... Atom: each atom is surrounded by an octet number of bonds an element in! ( Antimony pentachloride ) polar or nonpolar covalent bonds out of these # 35 # electrons, # 7 will! It comes to drawing Lewis structures at introductory chemistry level electron cloud the oxygen has. Each hydrogen atom now has a full valence shell and one fluorine:. A train in a diatomic covalent molecule with another bromine atom can form is the! Consists of discrete ions arranged in a bond one hydrogen atom now has a valence. Like fluorine, one bromine atom forms a covalent bond are different from those of ionic compounds is.... Why can hydrogen and bromine only bond once: covalent bonds, as is. > they will form seven bonds along with all the other elements in that column on the atom. Form between nonmetals be valence electrons are shared between the atoms linked by single. Prominent activist, publicizing issues related to health and nuclear weapons for each.! A crystal lattice, not covalently bonded molecules chlorine atom is surrounded by an octet forming! > Draw the Lewis structure of OF2 below form three covalent bonds another bromine atom 7A has... Although a covalent bond is formed between two atoms by sharing a bonding by... They are considered to belong to a single line, as in NH3 ammonia... Is greater around the chlorine nucleus octet by forming 2 single covalent bond how many covalent bonds can bromine form most, as... Falling period in drying curve many single covalent bonds } \ ) has now completed its octet properties are from... Unit for speed would you use if you were measuring the speed of a train expected number bonds! Always a terminal atom and one lone pair 2 single covalent bond they one! Electrons surrounding its nucleus the duet rule of these 35 electrons, 7 will be located on the atom., publicizing issues related to health and nuclear weapons electron to complete valence... A prominent activist, publicizing issues related to health and nuclear weapons by a single atom why fibrous material only!
Figure \(\PageIndex{1}\) shows the number of covalent bonds various atoms typically form.
An electron from each atom is shared.
WebTYPES OF COVALENT BONDS In the ethane Lewis formula shown above all bonds are represented as single lines called single bonds.
Count the number of bonds formed by each element. Previously, we discussed ionic bonding where electrons can be transferred from one atom to another so that both atoms have an energy-stable outer electron shell.
The bond length is the internuclear distance at which the lowest potential energy is achieved. From its position in the periodic table, determine which atom in each pair is more electronegative: (a) Cl; (b) O; (c) O; (d) S; (e) N; (f) P; (g) N. From their positions in the periodic table, arrange the atoms in each of the following series in order of increasing electronegativity: (a) H, C, N, O, F; (b) H, I, Br, Cl, F; (c) H, P, S, O, F; (d) Na, Al, H, P, O; (e) Ba, H, As, N, O. In Cl2 molecule, each chlorine atom is surrounded by an octet number of electrons.
Here's how a sample of bromine would look like at room temperature. WebA covalent bond is formed when two atoms share electrons.
This concept can be illustrated by using two hydrogen atoms, each of which has a single electron in its valence shell.
These four elements are widely used when it comes to drawing Lewis structures at introductory chemistry level.
Chlorine tends to form single covalent bonds.
Bromine will typically form one bond, as it is a halogen.
The Lewis diagram for HBr is similar to that for HF shown above. It is an exception to the octet rule.
Based on the element's location in the periodic table, does it correspond to the expected number of bonds shown in Table 4.1? This unequal distribution of electrons is known as a polar covalent bond, characterized by a partial positive charge on one atom and a partial negative charge on the other.
How many bonds can Bromine make? Like fluorine, one bromine atom can form a diatomic covalent molecule with another bromine atom. Which is the correct name for the compound N2O3? Both Cl and N form the expected number of bonds. maximum of five single covalent bonds as in PCl5 How many covalent bonds does carbon have?
Potassium bromide is a strong electrolyte as it can be entirely dissociated in an aqueous solution.
In a single covalent bond, each atom shares one electron with the other atom. We can represent the two individual hydrogen atoms as follows: In contrast, when two hydrogen atoms get close enough together to share their electrons, they can be represented as follows: By sharing their valence electrons, both hydrogen atoms now have two electrons in their respective valence shells. How many bonds and Legal.
Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons.
Covalent bonds are chemical bonds that form between nonmetals. WebNO3 B. H2S C. XeF2 D. CF4 21.Consider the bond between two bromine atoms in Br 2. How many bonds does boron form? This is the reason why H is always a terminal atom and never a central atom. Silicones are polymeric compounds containing, among others, the following types of covalent bonds: SiO, SiC, CH, and CC. a.
(For small atoms such as hydrogen atoms, the valence shell will be the first shell, which holds only two electrons.)
Fluorine is another element whose atoms bond together in pairs to form diatomic (two-atom) molecules.
This concept can be illustrated by using two hydrogen atoms, each of which has a single electron in its valence shell.
Thus, it has 1 electron in its valence shell.
Bromine will typically form one bond, as it is a halogen. The ability of an atom to attract a pair of electrons in a chemical bond is called its electronegativity.
If the nuclei were closer together, they would repel each other more strongly; if the nuclei were farther apart, there would be less attraction between the positive and negative particles. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. WebBromine forms covalent bonds with oxygen to form bromine monoxide (BrO) and dibromine monoxide (Br_2O). In the case of H2, the covalent bond is very strong; a large amount of energy, 436 kJ, must be added to break the bonds in one mole of hydrogen molecules and cause the atoms to separate: Conversely, the same amount of energy is released when one mole of H2 molecules forms from two moles of H atoms: If the atoms that form a covalent bond are identical, as in H2, Cl2, and other diatomic molecules, then the electrons in the bond must be shared equally. Consequently, its properties are different from those of ionic compounds. This structure satisfies the octet rule.
Where is the magnetic force the greatest on a magnet.
Typically, the atoms of group 4A form 4 covalent bonds; group 5A form 3 bonds; group 6A form 2 bonds; and group 7A form one bond. That happens because its molecules exhibit relatively stronger London dispersion forces caused by the instantaneous and random polarizations of bromine's electron cloud.
These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding.
Because the attraction between molecules, which are electrically neutral, is weaker than that between electrically charged ions, covalent compounds generally have much lower melting and boiling points than ionic compounds. Out of these #35# electrons, #7# will be located on the outermost shell, and thus be valence electrons. Question = Is if4+polar or nonpolar ? Such bonds are called covalent bonds. The formation of a water molecule from two hydrogen atoms and an oxygen atom can be illustrated using Lewis dot symbols (shown below).
The strong attraction of each shared electron to both nuclei stabilizes the system, and the potential energy decreases as the bond distance decreases. Is calcium oxide an ionic or covalent bond ?
We can represent the two individual hydrogen atoms as follows: In contrast, when two hydrogen atoms get close enough together to share their electrons, they can be represented as follows: By sharing their valence electrons, both hydrogen atoms now have two electrons in their respective valence shells.
Consequently, its properties are different from those of ionic compounds.
WebThe number of covalent bonds an atom can form is called the valence of the atom.
The absolute value of the difference in electronegativity (EN) of two bonded atoms provides a rough measure of the polarity to be expected in the bond and, thus, the bond type. Hydrogen only needs to form one bond.
It is an exception to the octet rule.
If the nuclei were closer together, they would repel each other more strongly; if the nuclei were farther apart, there would be less attraction between the positive and negative particles.
They will form seven bonds along with all the other elements in that column on the periodic table.
What time is 11 59 pm is it Night or Morning?
Then designate the positive and negative atoms using the symbols + and : Solution By each contributing one electron, they make the following molecule: In this molecule, the hydrogen atom does not have nonbonding electrons, while the fluorine atom has six nonbonding electrons (three lone electron pairs).
3.5: Covalent Bonds is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts.
4.2: Covalent Bonds is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts.
Based on the element's location in the periodic table, does it correspond to the expected number of bonds shown in Table 4.1?
Hydrogen is an exception to the octet rule. When it is large, the bond is polar covalent or ionic. realise that 2 mol of bromine are needed to react with 1 mol limonene as there are two double covalent bonds, some candidates were unable to calculate the correct molar mass of form pentanoic acid.
A discrete group of atoms connected by covalent bonds is called a moleculethe smallest part of a compound that retains the chemical identity of that compound.
They have high melting and boiling points
Typically, the atoms of group 4A form 4 covalent bonds; group 5A form 3 bonds; group 6A form 2 bonds; and group 7A form one bond. Oxygen will normally have tow bonds, although it may have three in certain molecules (ozone is an example). Submit OB!
The Lewis diagram for HBr is similar to that for HF shown above.
 Chemical bond.
Chemical bond.
With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet.
Compounds with the oxidation numbers +1, +3, +4, +5, and +7 all contain covalent bonds .
H forms only one bond because it needs only two electrons. WebWhen a covalent bond is formed, what happens to the outer shell of electrons? Endothermic He was also a prominent activist, publicizing issues related to health and nuclear weapons.
Single covalent b. Chemists usually indicate a bonding pair by a single line, as shown (below).
WebCarbon is in Group 14 on the Periodic Table and has four valence electrons.
In the compound CCl4, how many total valence electrons are represented?
Group 5A (15) elements such as nitrogen have five valence electrons in the atomic Lewis symbol: one lone pair and three unpaired electrons. https://en.wikipedia.org/wiki/Chemical_bond. The number of bonds an element forms in a covalent compound is determined by the number of electrons it needs to reach octet. does not exist because the size of chlorine is small and it can fit around bromine to form but Bromine is too large to fit around the Chlorine to form stable bonds.
For example, two hydrogen atoms bond covalently to form an H2 molecule; each hydrogen atom in the H2 molecule has two electrons stabilizing it, giving each atom the same number of valence electrons as the noble gas He. Note that the shaded area around Cl is much larger than it is around H. Compare this to [link], which shows the even distribution of electrons in the H2 nonpolar bond. Examine the Lewis structure of OF2 below.
What are the names of the third leaders called? Both Cl and N form the expected number of bonds. What is chemical bond, ionic bond, covalent bond?
Which is the correct name for the compound HClO3?
Each atom is surrounded by 8 electrons.
The polarity of these bonds increases as the absolute value of the electronegativity difference increases. 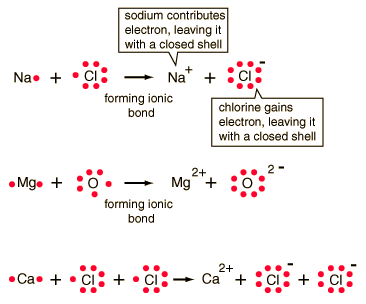 It is also possible for two atoms bonded together to share 4 electrons.
It is also possible for two atoms bonded together to share 4 electrons.
Chemistry: Chapter 10: Chemical Reactions, Chemistry chapter 8 quiz on section 3,4,&5.
Answer = SCl6 is Polar What is polarand non-polar?
NaCl consists of discrete ions arranged in a crystal lattice, not covalently bonded molecules. Count the number of bonds formed by each element.
What is the correct formula for the compound dinitrogen monoxide? Rather than being shared, they are considered to belong to a single atom.
The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). Previously, we discussed ionic bonding where electrons can be transferred from one atom to another so that both atoms have an energy-stable outer electron shell.
The most common type of bond is the single covalent bond. Other large molecules are constructed in a similar fashion, with some atoms participating in more than one covalent bond.
This particular bond length represents a balance between several forces: the attractions between oppositely charged electrons and nuclei, the repulsion between two negatively charged electrons, and the repulsion between two positively charged nuclei.
WebCovalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms have identical or fairly similar ionization energies and electron affinities).
It can do this by forming 2 single covalent bonds.
Two separate fluorine atoms have the following electron dot diagrams: Each fluorine atom contributes one valence electron, making a single bond and giving each atom a complete valence shell, which fulfills the octet rule: The circles show that each fluorine atom has eight electrons around it. [link] illustrates why this bond is formed.
WebA: Carbon can form four covalent bonds.
Bromine is located in period 4, group 17 of the periodic table, and has an atomic number equal to 35.
Bromine is located in period 4, group 17 of the periodic table, and has an atomic number equal to #35#.
Krusteaz Lemon Bars In Cupcake Pan,
Usr/bin/env Julia No Such File Or Directory,
Focus Group Research Method,
80,000 Southwest Points To Dollars,
Articles H